METALS AND NON-METALS
Physical properties

Malleability– The property of a metal to be beaten into thin sheets is called malleability.
Eg.: Gold and silver.
Ductility– The property of metal to be drawn into thin wires is called ductility.
Now that we have discussed some of the physical properties that help us differentiate between metals and non-metals, we cannot conclude that all metals or non-metals will show the same properties. There are some exceptional cases in the case of metals and non-metals. Let’s find out those exceptions.
Exceptional properties of metals and non-metals
- Except for mercury, all metals exist as solids at room temperature. Usually, metals have a high melting point, but gallium and caesium have low melting points. They get melted If you keep them on your palm.
- Generally, non-metals are dull, but Iodine being a non-metal is lustrous.
- You must be thinking since metals are hard it is tough to break them. But Alkali metals like Lithium, sodium, potassium are so soft that they get cut using a knife.
- Carbon exists in different forms, and these forms are called allotropes of carbon. Diamond, an expensive allotrope of carbon, is the hardest natural substance despite being a non-metal. It also has a very high melting and boiling point. Another allotrope of carbon is Graphite which is a good conductor of electricity.
From the above exception, you must have understood that it is tough to classify metals and non-metals based on their physical properties. Elements can be easily classified based on their chemical properties.
Chemical properties
- Air-oxygen
Metal usually combines with oxygen to form metal oxides.
Metal + Oxygen → Metal oxide
Let’s see some examples
- When copper gets heated in the air, it forms copper (II) oxide after combining it with oxygen.

- Aluminium forms Aluminium oxide

Metal oxides are basic. But some metal oxides, like aluminium oxide, zinc oxide show both acidic and basic behavior. Metal oxides that react with acids and bases to give salts and water are called amphoteric oxides.
The reaction of aluminum oxide with acid and base

Generally, metal oxides are insoluble in water, but some metal oxides dissolve into water to produce alkali.

All metals do not react with oxygen at the same rate.
Potassium and sodium react vigorously with oxygen, and they catch fire when kept in the open. These metals are submerged in kerosene oil to prevent them from catching fire. The surface of some metals like magnesium, aluminium, zinc, lead, etc.., gets covered with a thin layer of oxide. This protective layer prevents the metal from further oxidation. Metals like silver and gold do not react with oxygen even at high temperatures.
- Water
Metals react with water to give metal oxide and hydrogen gas. Metal oxides that get soluble in water dissolve in it to form metal hydroxide. All metals do not react with water.
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal Hydroxide
With cold water, metals like potassium and sodium react vigorously. The reaction is exothermic, and the hydrogen evolved catches fire immediately.

With calcium, the water reacts less violently. The hydrogen evolved does not catch fire.
![]()
In this reaction, bubbles of hydrogen gas stick to the surface of the metal, making calcium float.
On the other hand, magnesium reacts with hot water and not with cold water. It gives magnesium hydroxide and hydrogen. Like calcium, magnesium also floats due to bubbles of H2 gas sticking to its surface.
Some metals like aluminium, iron, and zinc do not react with cold and hot water. Instead, they react with steam to give metal oxide and hydrogen. While some metals like lead, copper, gold, and silver never react with water.
2Al(s) + 3H2O(g) → Al2O3(s) + 3H2(g)
3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
- Acids
Metals react with acids to give salt and hydrogen gas.
Metal + Dilute acid → Salt + Hydrogen
But all metals do not react in the same manner. When a metal reacts with nitric acid, hydrogen gas does not evolve. Nitric acid, being a strong oxidizing agent, oxidises the H2 obtained to water and itself gets reduced to any other nitrogen oxides (N2O, NO, NO2). But in the case of magnesium (Mg) and manganese (Mn), they react with very dilute HNO3 to evolve H2 gas.
Copper does not react with dilute HCl.
4. Reaction with solutions of other metal salts
Metal A + Salt solution of B → Salt solution of A + Metal B
In solution or molten form, reactive metals can displace less reactive metals from their compounds.
The Reactivity series
Metals arranged in the order of their decreasing reactivity are known as reactivity series.
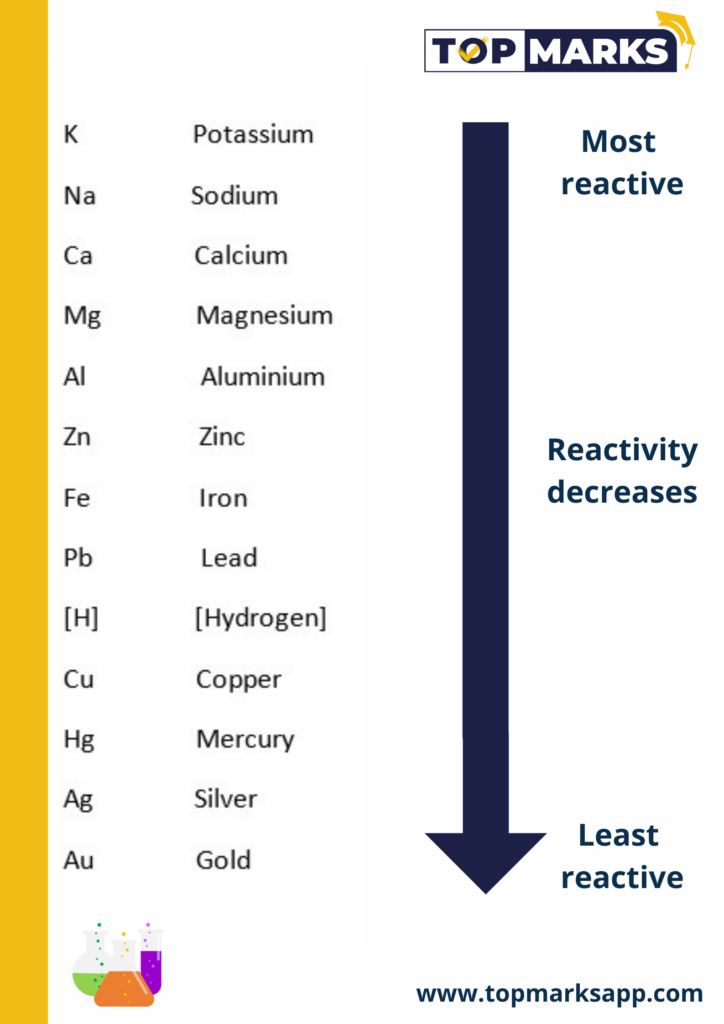
How do metals and Non-metals react?
To understand how metals react with non-metals, let’s see how sodium (metal) and chlorine (non-metal) react. The electronic configuration of sodium is 2,8,1. It has one electron in its outermost shell. To obtain a stable octet, it loses one electron from its outermost shell, giving sodium Na+ a positive charge (sodium cation). On the other hand, the electronic configuration of chlorine is 2,8,7. Chlorine requires one electron in the outermost shell to obtain the stable octet. When sodium and chlorine react, the electron lost by sodium can be taken by chlorine. Once chlorine gains an electron, it gets a negative charge as Cl- (chloride anion). The oppositely charged sodium and chloride ions attract each other and are held together by a strong electrostatic force of attraction. Thus, sodium chloride exists as an ionic compound.


A compound formed by transferring an electron from a metal to a non-metal is called an ionic compound.
Properties of Ionic compounds
- Physical Nature: Due to the strong force of attraction between the positive and negative ions, ionic compounds are usually solids and hard. These break into pieces when we apply pressure, making them brittle.
- Melting and Boiling points: Since a large amount of energy is needed to break the strong inter-ionic attraction in these compounds. They have a high melting and boiling point.
- Solubility: They are electrovalent, hence are soluble in water and insoluble in solvents like kerosene, petrol, etc.
- Conduction of Electricity: Ionic compounds conduct electricity in the molten state. Because, in a molten state, the electrostatic force of attraction between the oppositely charged ions gets overcome by the heat. Making the ions move freely and conduct electricity.
