Chemical Reactions and EquationsClass 10 Notes
Chemical reactions are the process by which new substances with new properties are formed, or in other words, when chemical changes of matter occur, chemical reactions take place. During chemical reactions following changes occurs:
- Change in colour: Reaction of iron and copper sulphate. In this reaction blue coloured copper sulphate solution turns light green when we add the iron.
- Change in state: Ammonia gas reacts with hydrogen chloride gas to produce solid ammonium chloride.
- Evolution of gas: When zinc reacts with hydrochloric acid, hydrogen gas is evolved with formation of zinc chloride.
- Change in temperature: Burning fire produces heat.
Chemical reactions in our daily life
- Rust
- Digestion
- Soap and Detergent Reactions
- Photosynthesis
Chemical equations
We can represent the chemical reactions using chemical formulae and symbols.
- The left-hand side of a chemical equation represents the reactants, and the right-hand side represents the products.
- An arrow marks that points towards the products side shows the direction of the reaction.
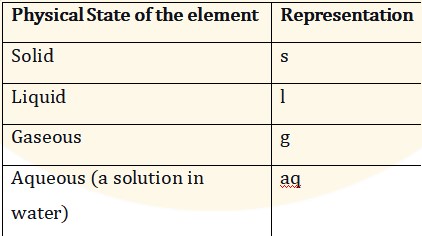
- The physical state of chemicals is denoted using the following
- (s) for solid.
- (l) for liquid.
- (g) for gas.
- (aq) for aqueous solution.
How to balance a chemical reaction?
Balanced chemical equation is an equation in which the number of atoms for each element on the reactant side and product side is equal.
Why we need to balance the equation?
We need to balance chemical equation because of law of conservation of mass. It states that ‘matter can neither be created nor be destroyed.
Let us see the steps to be followed for writing a balanced chemical equation
Fe + H2O → Fe3O4 + H2
Step 1: To balance a chemical equation, draw boxes around each formula.
\boxed{Fe}+\boxed{H_2O}\rightarrow \boxed{Fe_3O_4} + \boxed{H_2}
Step 2: Now write down the number of atoms of different elements present in the unbalanced equation i.e., here we list out the number of atoms of Iron (Fe), Hydrogen (H), and Oxygen (O).
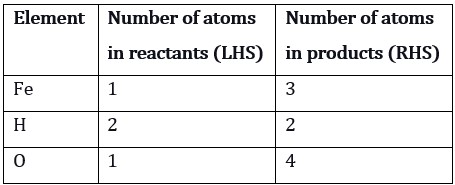
Step 3: For balancing a chemical equation, it is more convenient if we start balancing with the compound that contains the maximum number of atoms. It can be a reactant or a product. If we consider this criterion, we take Fe3O4 and the element oxygen. There are four oxygen atoms on the product side and only one on the reactant side. To balance the oxygen atoms on both sides-

You should keep in mind that while making the number of atoms equal, we cannot alter the formula of the compound or elements involved in the reaction. The partially balanced equation becomes-
\boxed{Fe}+4\boxed{H_2O} \rightarrow \boxed{Fe_3O_4}+\boxed{H_2}
Step 4: To proceed further, we need to balance Fe and H atoms. Balance the hydrogen atoms in the partly balanced equation. Here, to equalize the number of hydrogen atoms, we have to make the number of molecules of hydrogen as four on the product side.

Now the equation would be-
\boxed{Fe}+4\boxed{H_2O} \rightarrow \boxed{Fe_3O_4}+4\boxed{H_2}
Step 5: Both oxygen and hydrogen atoms, the only element left to be balanced is Iron (Fe). Now that we have balanced.
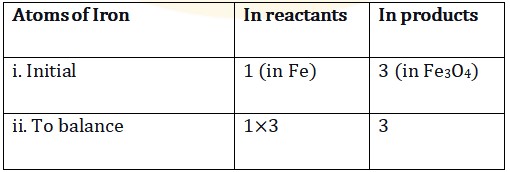
To make the iron atoms equal on both sides, we take three atoms of Fe on the reactant side.
3\boxed{Fe}+4\boxed{H_2O} \rightarrow \boxed{Fe_3O_4}+4\boxed{H_2}
Step 6: At the end make sure that the equation is balanced. This you can check by counting the atoms of each element on both sides of the equation is equal.
3Fe + 4H_2O → Fe_3O_4 + 4H_2
You can see that
- The number of atoms of Iron (Fe) is 3 on both sides
- The number of atoms of oxygen (O) is 4 on both sides
- And that of hydrogen is 8 on both sides
Thus, we get a balanced chemical equation. This method is also known as hit- and -trial method as we make trials to balance the equation.
Step7: We must write the symbols of the physical states of each element in the equation. To make the equation more informative, the physical states of the reactant and product are mentioned along with their chemical formulae.
The balanced equation is-
\boxed{3Fe(s) + 4H_2O(g) → Fe_3O_4(s) + 4H_2(g)}
Here you can see that the symbol (g) is used for H2O. This indicates that in this reaction water is used in the form of steam. Unless it is necessary to specify the physical states of the elements, they are not included in a chemical equation. In some cases, to show the reaction condition like temperature, pressure, catalyst, etc.., they are indicated above and/or below the arrow in the equation.
Types of chemical reactions
Combination Reaction
A chemical reaction in which two or more compounds react to give a new product.
Let’s see some everyday reactions to understand this-
- Burning of coal: C(s) + O2(g) → CO2(g)
- Formation of water from H2(g) and O2(g): 2H2O + O2(g) → 2H2O(l)
Combination reaction is a reaction in which two or more substances (compound or elements) combine to give a single product.
Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. Examples of exothermic reactions are:
- Formation of calcium hydroxide (slacked lime): CaO(s) + H2O(l) → Ca (OH)2(aq) + Heat. Here we can see that Calcium oxide reacts with water to form a single product Calcium Hydroxide.
Respiration is a combination reaction. We, humans, require energy to survive and we receive it from the food we consume. During digestion, food is broken down into simpler substances. Food items like rice, potatoes contain carbohydrates. These are broken down into glucose. This glucose combines with oxygen in the cells of our body and provides energy. This process is called respiration and can be represented as: C6H12O6(aq) + 6O2(aq) → 6CO2(aq) + 6H2O(l) + energy
Decomposition reaction
A chemical reaction in which a single compound breaks down into two or more elements or creates a new compound is known as decomposition reaction. These reactions require an energy source such as heat, light, or electricity to break the bonds of compounds. Ex: 2FeSO4(s) Fe2SO4(s) + SO2(g) + SO3(g)
In the above reaction Ferrous sulphate crystals (FeSO4.7H2O) on heating lose water. Thus, it decomposes to give ferric oxide (Fe2O3), Sulphur oxide (SO2), and sulphur trioxide (SO3). The colour of the crystals changes on heating.
Some of decomposition reactions:
- Decomposition of Calcium carbonate to calcium oxide and carbon dioxide. CaCO3(s)CaO(s) + CO2(g). This reaction is used in various industries. The calcium oxide (quick lime) formed is extensively used in the manufacture of cement. Since the decomposition reaction is carried out by heating, it is called thermal decomposition.
- Decomposition of silver chloride into silver and chlorine. 2AgCl(s) 2Ag(s) + Cl2(g). Here the white silver chloride (AgCl) turns grey in the presence of sunlight. This is due to the decomposition of AgCl to Silver (Ag) and chlorine (Cl2) by sunlight.
- Decomposition of silver bromide to silver and bromine. Silver bromide also reacts in the same way as silver chloride reacts with sunlight. 2AgBr(s)2Ag(s) + Br2(g). This reaction is used in black and white photography. A reaction in which energy is absorbed is called an endothermic reaction.
Displacement reaction
A displacement reaction refers to a reaction in which a part of one reactant is displaced by another reactant in a compound. Fe(s) + CuSO4(aq) → FeSO4(aq) +Cu(s) is an example for this reaction.
In the above reaction, iron has displaced copper from copper sulphate solution. Here, the more reactive element iron (Fe) displaces a less reactive element copper from its copper sulphate solution.
Some other examples of displacement reactions:
- Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
- Pb(s) + CuCl2(aq) → PbCl2(aq) + Cu(s)
When compared to copper, zinc and lead are more reactive elements. Hence, they displace copper from its compounds. Thus, we can conclude that in a displacement reaction, a more reactive element displaces a less reactive element from its compound.
Double Displacement reaction
A double displacement reaction takes place when two chemicals react and the positive ions (cation) and negative ions (anion) of the two reactants exchange positions. When the solution of sodium sulphate is mixed with barium chloride, a white substance is formed. This white substance which is insoluble in water is called a precipitate. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + 2NaCl(aq). Here, the reaction is ionic.
When the reactants are dissolved in water, they become ions and the ions get exchanged in the solution. The Ba2+ and SO42- ions react to give the white precipitate of BaSO4. The NaCl is formed by the reaction of Na2+ and Cl- ions remain in the solution. Thus, it gives a precipitate of barium sulphate in an aqueous solution of sodium chloride.
Oxidation and Reduction
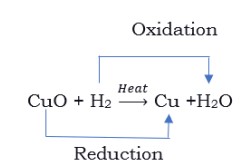
Let’s consider a reaction in which copper powder is heated in a China dish: 2Cu +O2 2CuO. During this reaction, the oxygen reacts with copper to give a black coating of copper (II) oxide. This black colour is due to the reaction of oxygen.
When hydrogen is passed over this copper oxide (CuO), the black coating turns brown. Thus, the reverse reaction takes place and copper is formed again. CuO + H2 Cu + H2O
In the first reaction, the substance copper gains oxygen i.e., copper is oxidised. In the second reaction, the copper (II) oxide on reaction with hydrogen loses oxygen i.e., copper oxide is reduced. The hydrogen is gaining oxygen and is oxidised.
The reactions in which oxidation (loss of electrons) and reduction (gain of electrons) take place simultaneously are called redox reactions.
Oxidation is the gain of oxygen or loss of hydrogen. Reduction is the loss of oxygen or gain of hydrogen.
CORROSION
Have you seen that iron articles when bought new are shiny, but as time goes they get coated with reddish brown powder. This is due to the process of rusting of iron.
When metals come in contact with substances like water or air, the pure metal gets converted to undesirable substances. This process is called corrosion. Other examples of corrosion are the black coating on silver and the green coating on copper.
Corrosion causes destruction to car bodies, bridges, iron railings, ships and all objects that are made of metals. Iron corrosion is a severe problem as every year a large amount of money is spent to replace damaged iron.
RANCIDITY
Have you observed that when food containing oil/fat is kept outside for a long time, it gives an unpleasant smell and taste. This is due to rancidity.
When fats and oils in food undergo oxidation, their smell and taste changes. This process is called rancidity.
Ways to prevent rancidity
- Antioxidants (substances which prevent oxidation) are added to food.
- Keep food in air tight containers.
- Gas like nitrogen can be filled in chips packets to prevent oxidation.
Some more important concepts
\rArrBalanced chemical equation for process of photosynthesis is given below: \\6CO_2(g)+6H_2O(l) \xrightarrow{sunlight} C_6H_{12}O_6(s) + 6O_2(g).
\rArrBurning of natural gas: \\CH_4(g) + 2O_2 (g) → CO_2 (g) + 2H_2O(l) + heat + energy
\bold \rArrSome more combination reactions: \\ \text{Magnesium wire is burnt in air:} 2Mg(s) + O_2 \rightarrow 2MgO(s). \text{This reaction is also a redox reaction.} \\NH_3(g)+HCl(g) \rightarrow NH_4Cl(s)
\bold \rArrSome more Decomposition reaction\\2FeSO_4(s) \xrightarrow{heat} Fe_2O_3(s)+SO_2(g) + SO_3(g). \text{In this reaction Green colour of} FeSO_4 \text{disappears and reddish brown solid is formed.}
\bold \rArrImportance of decomposition reaction in metal industry
- Molten NaCl is electrolytically decomposed to form sodium metal.
- Aluminum metal is obtained by electric decomposition of bauxite ore mixed with cryolite.
- Carbonate ores are thermally decomposed to give metal oxide which on reduction give metal.
