Carbon and it's compounds
Bonding in Carbon
Now that you know how crucial carbon is for human survival on our planet, let’s look at the features of carbon compounds.
- They are poor conductors of electricity
- Low Melting point and Boiling point gives us the conclusion that the force of attraction between the molecules is weak
As you all know, the atomic number of carbon is 6. The outermost shell of carbon has four electrons. To attain noble gas configuration, carbon has to gain or lose four electrons from its outermost shell. But this is not an easy task for carbon. If it gains four electrons becoming a C4- anion, the nucleus with six protons has to hold ten electrons. On the other hand, if it loses four-electron to form a C4+ cation, the nucleus with six protons will hold on to two electrons. Carbon overcomes this difficulty by sharing its valence electron with other carbon atoms or atoms from other elements.
Such bonds exist in some simple molecules like H2, O2, and N2. The atomic number of hydrogen is 1. The K shell has one electron. A molecule of hydrogen, H2 is formed when two hydrogen atoms share their electrons, thus attaining noble gas configuration. The valence electrons are represented using dots or crosses. The single covalent bond between the hydrogen atoms is represented by a line between them. Fig.
Now we will see a carbon compound that is used as fuel -Methane. It has a formula of CH4. Carbon has four valence electrons and hydrogen has one valence electron. So, to achieve the noble gas configuration carbon shares its 4 electrons with four atoms of hydrogen. Fig.
The bonding in carbon is Covalent. A covalent bond is a chemical bond in which the electrons of the atoms are shared equally. Here the electrons in the outermost shell are shared. Such molecules have strong bonds within the molecule, but the intermolecular forces are weak. This is the reason for the low boiling and melting point of such compounds. These covalent compounds are poor conductors of electricity as the electrons are shared between atoms and no charged particle is formed.
Versatile Nature of Carbon
- Catenation
Carbon can form bonds with other atoms of carbon, which give rise to large molecules. This property of carbon is called catenation. Such compounds have long chains of carbon, branched chains of carbon or carbon atoms arranged in rings. Carbon atoms may also be linked through single, double, or triple bonds. Carbon compounds linked by single bonds are called saturated compounds. Those carbon compounds which are linked through double or triple bonds are called unsaturated compounds.
2. Tetravalency
Carbon has a valency of four and can bond with four other carbon atoms or atoms of other mono-valent elements. Carbon forms a compound with hydrogen, oxygen, nitrogen, sulphur, chlorine, and many other elements. These bonds are very strong making the compounds stable.
Saturated and Unsaturated Carbon Compounds
To make the structure of saturated or unsaturated carbon compounds, the first step is to link the carbon atoms with a single bond and then satisfy the remaining valencies of carbon using hydrogen atoms.
Let’s see the steps to arrive at the structure of ethane (C2H6)
Step 1-Carbon atoms are linked together with a single bond

Step 2-Each carbon atom is bonded to three hydrogen atoms to complete the carbon’s valency of four.

Fig- Electron dot structure of ethane
In compounds like methane, ethane, and propane the valencies of all atoms are satisfied by single bonds between them. These carbon compounds are called saturated compounds. They are normally not reactive.
Let’s see the steps to arrive at the structure of ethene(C2H4)
Step 1- carbon and carbon can be linked through a single bond.

Step-2- Each carbon atom is attached to two hydrogen atoms.
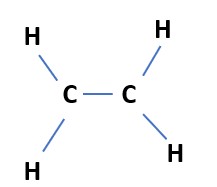
Step-3-Even after step 2 the valency of the carbon atom is not satisfied(valency-4), to satisfy the valency we need a double between the two carbon atoms.
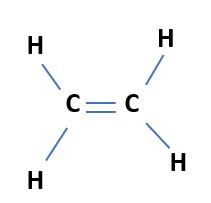
Fig- Electron dot structure of Ethene
Thus some compounds are formed using double or triple bonds between the carbon atoms. Such compounds are called unsaturated compounds. They are more reactive than saturated carbon compounds. Eg Ethene(C2H4) and ethyne (C2H2).
Chains, Branches, and Rings
Carbon compounds like methane, ethane, and propane contain 1,2 and 3 carbon atoms respectively. Such chains of the carbon atom can contain many more carbon atoms. Let’s look at some of these carbon chains.
Formulae and structures of saturated compounds of carbon and hydrogen

Branches
Consider the compound butane. It is possible to make two different carbon skeletons with four carbon atoms.

If we fill the remaining valencies with hydrogen, the following structures are formed with formula C4H10.
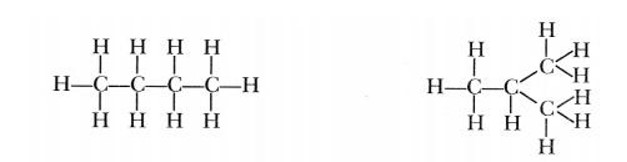
We can see that the above two structures have the same formula. Such compounds with identical molecular formulas but different structures are called structural isomers.
In addition to the straight and branched chains that you saw above, carbon atoms can be arranged in the form of a ring.
Example: Structure of cyclohexane
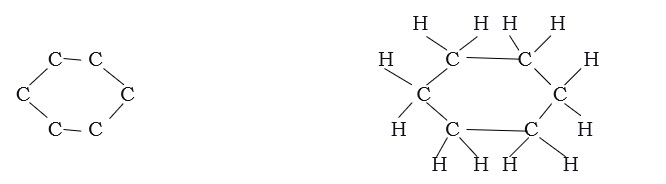
Saturated hydrocarbons with a single bond are called alkanes. The unsaturated hydrocarbons with a double bond and triple bond are called alkenes and alkynes respectively.
Some Functional Groups in carbon compounds
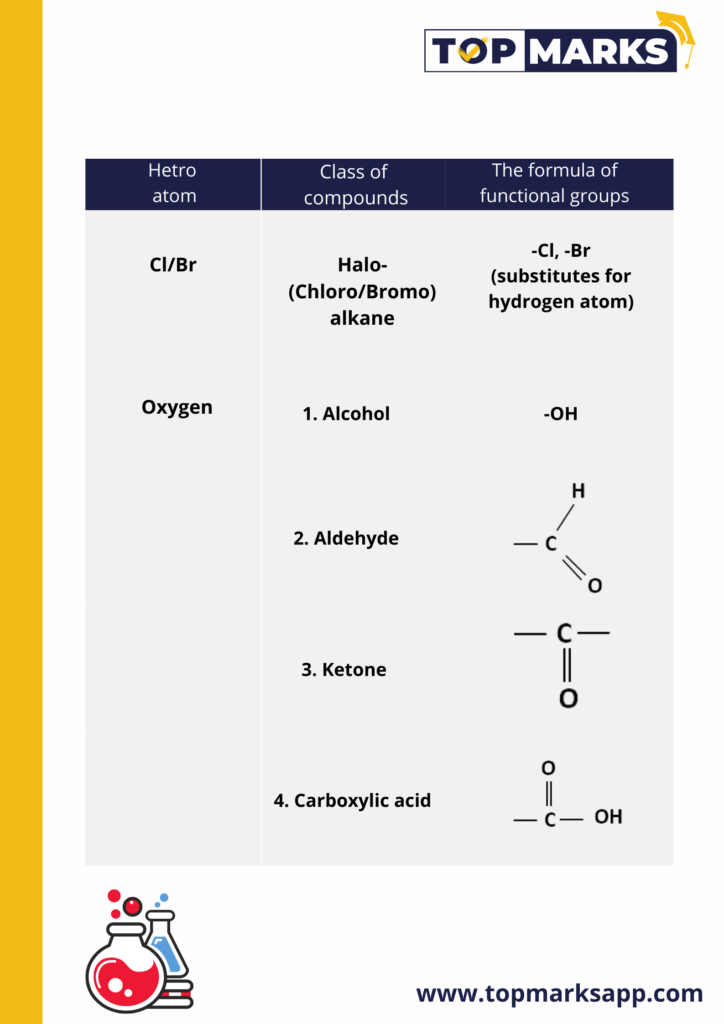
Homologous Series
We have already seen that carbon atoms can be linked to form carbon chains of different lengths. Some of these chains are branched too. In some cases, the hydrogen or other atoms attached to the carbon chain is replaced with any of the functional groups. And it is these functional groups that decide the properties of these carbon compounds. A series of compounds in which the same functional group substitutes for the hydrogen atom in a carbon chain is called a homologous series. For example, the chemical properties of CH3OH, C2H5OH, C3H7OH are very similar.
If we look at some formulas of the compound
- CH4 and C2H6 – they differ by a -CH2– unit
- C3H6 and C4H8– they differ by a -CH2– unit.
The chemical properties which are determined by the functional groups remain similar in a homologous series. The physical properties like melting point, boiling point, and solubility increase with increasing molecular mass.
- The general formula for Alkanes is CnH2n+2
- The gereral formula for Alkenes is CnH2n
- The general formula for Alkynes is CnH2n-2
Nomenclature of Carbon Compounds
Carbon compound can be named by the following method
- First, identify the number of carbon atoms in the compound. A compound having two carbon atoms would be named ethane.
- If the compound contains a functional group, it is indicated in the compound name with a prefix or suffix.
- If the functional group is given as a suffix, and the suffix begins with a vowel then the name of the carbon chain is modified by deleting the final ‘e’ and adding the appropriate suffix. For example, a three-carbon chain with a ketone group would be named in the following manner-Propan-‘e’ = propan‛one’ = propanone.
- If the carbon compound is unsaturated, then the final ‛ane’ in the name is replaced with ‛ene’ or ‛yne’.

Chemical Properties of Carbon Compounds
- Combustion
It is the process in which carbon burns in the presence of oxygen to give carbon dioxide along with the release of heat and light.
- C+O2 → CO2 + heat and light
- CH4+O2 → CO2 + H2O + heat and light
Saturated hydrocarbons give a clean flame while unsaturated compounds give a yellow flame with black smoke. But, if we limit the supply of air, this leads to incomplete combustion of saturated compounds giving sooty flame.
- Oxidation
Oxidation is the addition of oxygen to a compound. The combustion reaction that we just saw is an example of an oxidation reaction. Oxidation can also be defined as the removal of hydrogen, or loss of electrons in a chemical reaction.
Alkaline KMnO4 + Heat
CH3 – CH2OH → CH3COOH
Or acidified K2Cr2O7 + Heat
Here the substance alkaline KMnO4 or acidified K2Cr2O7 are capable of adding oxygen to the alcohol (ethanol). Thus, acting as oxidizing agents.
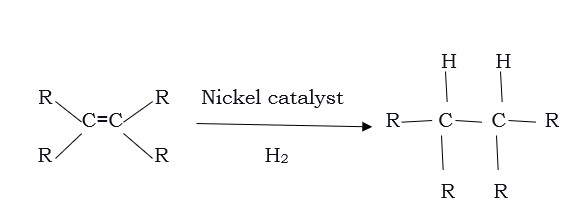
- Addition Reaction
It is the addition of hydrogen to unsaturated hydrocarbons in the presence of catalysts like palladium or nickel resulting in saturated hydrocarbons. Catalysts are a substance that causes or accelerates a chemical reaction without themselves being affected. In addition, the reaction is popularly used in the hydrogenation of vegetable oils using a nickel catalyst. Generally, vegetable oils have a long unsaturated carbon chain.
4. Substitution Reaction
Chlorine is added to hydrocarbons, in the presence of sunlight which leads to replacing of hydrogen atoms one by one. This is thus an example of substitution reaction. It is defined as the reaction in which one type of atom or group of atoms takes the place of another.
CH4+Cl2 → CH3Cl+HCl (in the presence of sunlight)
Some important carbon compounds- Ethanol and Ethanoic Acid
- Properties of Ethanol
- It is a liquid at room temperature
- An active ingredient of all alcoholic drink
- It is a good solvent- because of which it is used in medicine such as tincture iodine, cough syrups, and many tonics.
- It is soluble in water in all proportions
- Intake of a small quantity of pure ethanol (called absolute ethanol) is sufficient to cause death.
Reactions of Ethanol
1.Reaction with sodium
2Na+ 2CH3CH2OH → 2CH3CH2O− Na+ + H2
(Sodium ethoxide)
Hydrogen gas is evolved when sodium reacts with alcohol. Here, in addition to H2 gas, sodium ethoxide is also formed.
- Reaction to give unsaturated hydrocarbon– ethanol when heated at 443 K with excess concentrated sulphuric acid results in the dehydration of ethanol to give ethene.
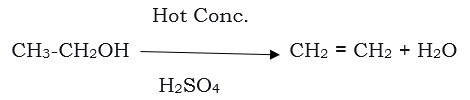
Here concentrated sulphuric acid acts as a dehydrating agent removing water from the ethanol.
- Properties of Ethanoic acid
- Ethanoic acid is popularly known as acetic acid.
- 5-8% of acetic acid in water is called vinegar which is used as a preservative in pickles.
- It is also known as glacial acetic acid- this is because it has a melting point of 290K and hence it freezes during winter.
Reactions of ethanoic acid
- 1. Esterification reaction
Esters are formed by the reaction of an acid and an alcohol. Ethanoic acid reacts with ethanol in the presence of an acid catalyst to give an ester-

Esters are sweet-smelling substances. They are used to make perfumes and as flavouring agents. On treating ester with sodium hydroxide, the ester is converted back to alcohol and sodium salt of carboxylic acid. This reaction is known as saponification. It is used in the preparation of soap. Sodium or potassium salts of long-chain carboxylic acid are called soaps.
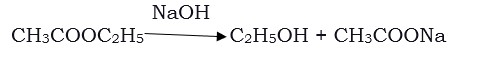
- Reaction with a base
Ethanoic acid reacts with a base such as sodium hydroxide to give sodium ethanoate (sodium acetate) and water.
![]()
- Reaction with carbonates and hydrocarbonates
The reaction of ethanoic acid with carbonates and hydrocarbonates gives rise to salt, carbon dioxide, and water.
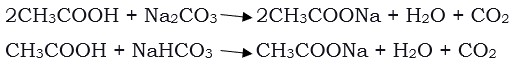
Soap and Detergents
As you all know, we use soaps and detergents to remove dirt from our clothes but there is an interesting mechanism behind this. Most dirt is oily in nature and oil does not dissolve in water. Soap molecules have two ends, one is hydrophilic, that is, it interacts with the water while the other end is hydrophobic, as it interacts with hydrocarbons. The ionic end (hydrophilic end) interacts with the water while the hydrophobic end interacts with oil. Thus, the soap molecules form a structure called micelles. In micelles, the hydrophilic end is towards the oil droplet while the hydrophobic end (ionic end) faces outside. As a result, an emulsion is formed in water. Thus, the soap micelle helps in removing the dirt from our clothes.
Fig. Micelle formation and how dirt is removed.
